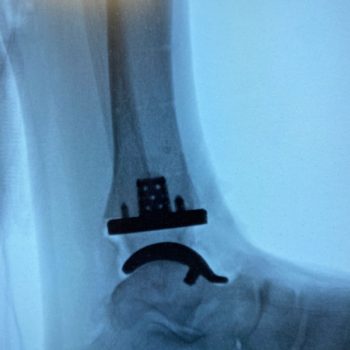
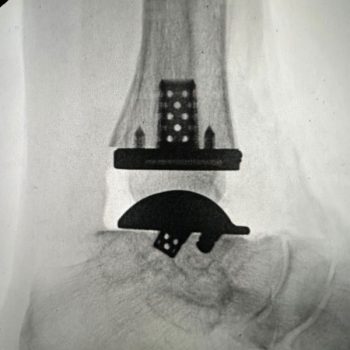
Created through additive manufacturing, the Vantage Ankle 3D and 3D+ tibias are designed to address a greater variety of ankle cases with simplified instrumentation.
Advita Announces First Total Ankle Surgeries with New 3D-Printed Tibial Implants

Gainesville, Fla (Sept. 25, 2024) – Advita, a global medical technology leader, announced today the successful completion of the first ankle replacement surgeries using its new Vantage® Ankle 3D and 3D+ tibial implants.
James Lachman, MD, of St. Luke’s University Health Network, performed the first ankle procedure last week in Easton, Penn.
“It is exciting to be the first to implant Advita’s latest ankle products and provide my patients with their differentiating benefits,” said Lachman. “The Vantage Ankle 3D+ prosthesis particularly stands out with its ability to address a greater variety of cases with simplified instrumentation.”
Advita’s Vantage Ankle 3D and 3D+ tibial implants provide surgeons with tibial stem heights from 10 to 30mm and the added benefits of 3D printing. Additive manufacturing creates a surface that mimics the trabecular nature of cancellous bone. Along with the 3D-printed surface, the implants also feature spiked pegs and a tall sharp central cage, with growing diameters, to aid with initial fixation.
...The Vantage Ankle 3D+ prosthesis particularly stands out with its ability to address more complex deformities with simplified instrumentation...
“Last week, nearly 8 years to the day following the first Vantage Ankle implantation, Jim Nunley and I are excited to have immediately followed Jim Lachman’s experience with two Vantage Ankle 3D+ total ankle arthroplasties at Duke,” Advita Design Team Surgeon Mark Easley, MD, said.
“After several years of developing the 3D+ tibial component with the outstanding Advita engineers and other design team surgeons, Jim, Jim [Lachman] and I are pleased how the new tibial components and instrumentation seamlessly melded with the existing talar component options. The additive manufacturing, and the press-fit pegs and augmented central cage afford satisfying initial tibial component stability. Particularly exciting is how the intuitive instrumentation allows for reliable and reproducible insertion of a stemmed tibial component through a routine anterior ankle surgical approach.”
These latest product additions follow a wide array of recent clearances and launches by Advita in the foot and ankle market. Earlier this year, the company launched a new advanced vitamin E polyethylene insert, Activit-E™, and is preparing to launch the world’s first ankle navigation system, AdvitaGPS® Ankle. In addition, Advita released additional patient-specific cutting guides, a collaboration with 3D Systems, that are compatible with the new implants.
Learn more about the Vantage Ankle 3D and 3D+ tibial implants and Advita’s full foot and ankle portfolio by visiting advita.com/ankle.
About Advita
Advita is a global medical technology leader that empowers orthopedic surgeons with innovative implants, surgical instruments and the Active Intelligence® (AI) ecosystem of smart technologies to give patients EXACTLY what they need to regain mobility. Visit advita.com for more information and connect with us on LinkedIn, Vumedi, YouTube, Instagram and X.
The Vantage Ankle PSI is manufactured by 3D Systems, Inc., and distributed by Advita in the U.S. AdvitaGPS is developed by Blue Ortho, an Advita subsidiary, and distributed by Advita, Inc.
Media Contacts:
Samantha DiVirgilio
Marketing Communications Sr. Manager
samantha.divirgilio@advita.com
Press & Media Contacts
Courtney Adkins
Marketing Communications Director
courtney.adkins@advita.com
Nancy Walsh
VP, Corporate & Marketing Communications
nancy.walsh@advita.com
Discover more
Sign up to receive email alerts with
the latest news, stories and updates.
Sign up to receive email alerts with the latest news, stories and updates.